
In part one Tom Denton shared his opinion that hydrogen is not an ideal fuel for cars and looked at how clean hydrogen is created. This time, he will examine briefly how a fuel cell converts hydrogen into electricity and then compare the efficiency of an EV and a FCEV.
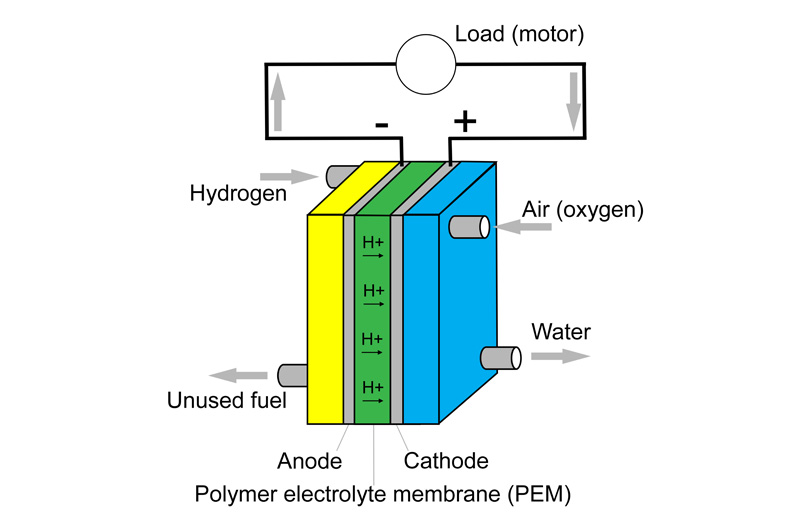
Proton-exchange membrane fuel cells, also known as polymer electrolyte membrane (PEM) fuel cells, are the type mainly used for vehicles. They produce electricity through the reaction of a fuel (hydrogen) with oxygen. Water is the only waste product from this type of fuel cell. The reaction between hydrogen and oxygen releases energy.
Fuel cells convert a substantial proportion of the chemical energy into electrical energy so are very efficient. All oxidations involve a transfer of electrons between the fuel and oxidant, and in a fuel cell this is employed to convert the energy directly into electricity.
The working temperature of fuel cells is 50-100°C, but some can be up to 200°C. High pressure is also used in the cell, in the region of 30 bar. A unit consisting of many fuel cells is referred to as a stack.
Individual cells are connected in series just like a normal battery. This forms a stack that meets the necessary voltage and current requirements. The theoretical open-circuit voltage of a hydrogen-oxygen fuel cell is 1.23V at room temperature, but in practice it is around 1V. Under load conditions, the cell voltage is between 0.5 and 0.8V. The stack voltage is usually 400-500V.
Opinion and facts
As mentioned previously, in my opinion, hydrogen is not the future for cars, but it will be used for storage and on larger vehicles such as buses and trucks; perhaps even for shipping and aviation.
Something that is often overlooked is that a hydrogen car is a battery electric vehicle – but with considerable added complexity (and therefore cost). FCEVs have a lithium-ion battery and all the other components that a standard BEV has, but they also have a fuel cell stack, fuel storage tanks and associated delivery systems.
For hydrogen to become a mainstream fuel, a new infrastructure will be required at fuel stations. The hydrogen will have to be created, compressed, delivered and stored. Unfortunately, hydrogen consumes a huge amount of energy as it is being created and compressed. Delivering electricity to a charger via the national grid is very efficient.
Creating and storing hydrogen when a wind turbine or solar farm is producing more electricity than is needed is an ideal solution. Truck refuelling stations could be located close to where this hydrogen is created to reduce delivery costs. However, to roll out a national network of hydrogen charging stations that rival petrol/diesel would be a huge task – some say we are struggling to roll out enough EV charge points, which is a much simpler process.

For me, the key information that we need to be aware of is how much electricity is needed to run a hydrogen powered vehicle. This image shows that the BEV is much more efficient:
Grid capacity will need to expand to charge more BEVs, but to create enough hydrogen for the equivalent FCEVs, the expansion would need to be more than double.
As a clean fuel, there is no doubt that hydrogen has exciting potential. The fact that big names like Bosch, VW, BMW, Toyota and others are investing in it definitely tells us something. However, there are a lot of technical challenges yet to solve, not least of which is the creation and distribution of hydrogen.
Hydrogen-powered cars have a lot going for them. They’re clean and offer good range. But on further examination, the amount of upstream electricity they use, makes them less attractive. Larger vehicles and local storage seem like good options, but we have all got a lot more to learn about the best way to use the benefits of hydrogen. Go BEVs!









